Nathan Pichot - April 23, 2024, Poitiers
Catalytic pyrolysis of solids
under the direction of Ludovic Pinard, Yannick Pouilloux and Anthony Dufour
Hydrocarbons (HC) are an irreplaceable resource in today’s world, being at the origin of products such as plastics, fuels, medicines and so on.
However, their production from fossil fuels contributes to resource depletion, pollution, the greenhouse effect and climate change.
It is therefore essential to diversify the sources of these compounds.
One promising method is biomass pyrolysis, which uses agricultural and forestry waste to produce oxygenated hydrocarbons (“pyrolysis oils”) and then, after a treatment stage on acid catalysts (“catalytic upgrading”), conventional HC.
The major obstacle to the commercialization of these processes is the rapid deactivation of the catalysts used.
It is therefore essential to study and remedy this phenomenon.
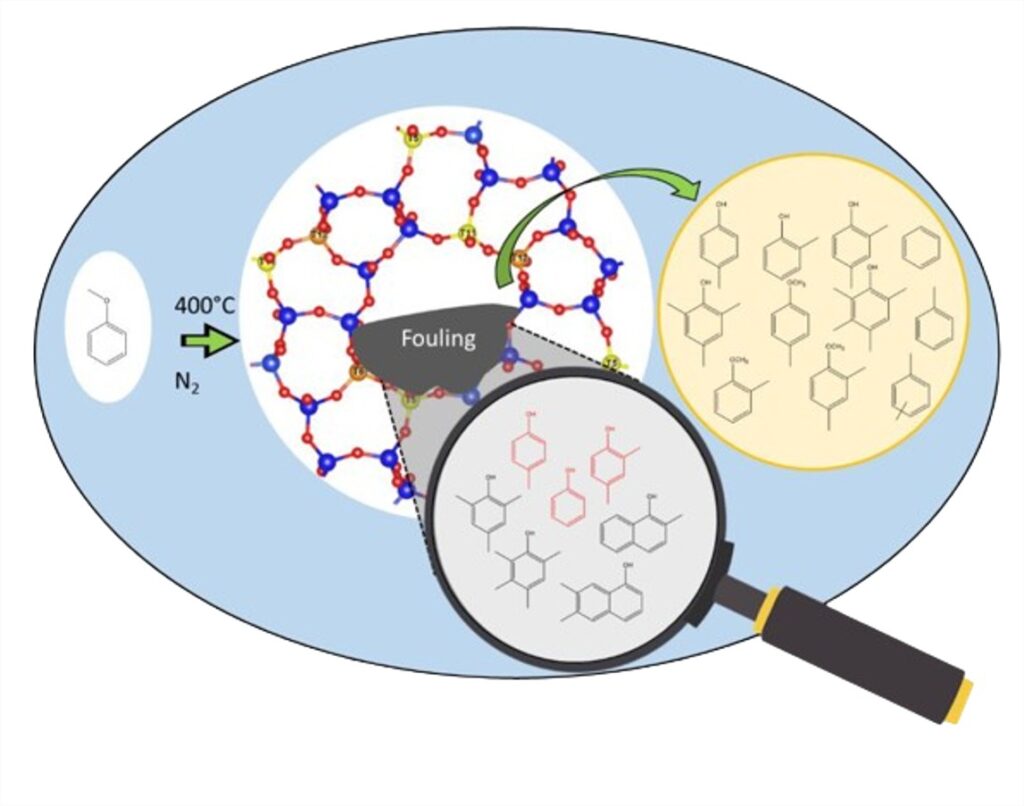
As the catalytic upgrading of pyrolysis oils is a complex process (numerous reagents and products), the work in this thesis focuses on the study of a model molecule, methoxybenzene (anisole), and its dismutation on acid catalysts, zeolites.
Anisole is an oxygenated aromatic molecule with a simple -OCH3 function substituted on itsC6 aromatic ring.
It is representative of some of the molecules derived from biomass pyrolysis, which possess this group.
The conversion of anisole on zeolites is a bimolecular dismutation reaction, involving the transalkylation of a methyl group from one anisole molecule to the aromatic ring of the other, forming one molecule of phenol and one of methylanisole.
Subsequent reactions form methylated phenolics (cresol, xylenol, etc.), which are notoriously deactivating for a catalyst, as they are easily adsorbed to its surface.
This thesis established the existence, after an initial rapid deactivation, of a state of stationary activity for anisole conversion.
Moreover, the phenolics produced by this conversion participate in catalyst deactivation, as expected, by adsorbing strongly onto the active sites, but also participate in stationary activity by being continuously available for possible transmethylation reactions with gas-phase reactants.
These phenolic compounds, strongly adsorbed on the catalyst surface, are found in the species extracted from the catalyst after reaction (coke), where “traditional” coke (heavy polyaromatic HC: pyrene, anthracene, etc.) is not detected.
The mesomeric effects induced by oxygenated groups (methoxy- , hydroxy-) inhibit the classic mechanism of formation of these polyaromatics by contraction-extension of methylated aromatic rings.
The effects of acidity (acid site density) and structure (MFI, *BEA, MOR, FAU, JZO) of the zeolites used on the phenomena presented above were investigated.
In the MFI zeolite (pore openings at 10 atoms T), the increase in the number of acid sites (low Si/Al) leads to a sharp decrease in the activity and rotational frequency of the catalyst’s active sites: the increase in the adsorption energies of the products (which can adsorb on two adjacent acid sites) induces an auto-inhibitory effect on the reaction.
This effect is found on zeolites with larger pores (*BEA, FAU, JZO), but is mitigated by the promotion of bimolecular reactions of the widest channels.
The auto-inhibition effect is also promoted by the low channel interconnectivity of 2D zeolites (MOR).
As these uncommon phenomena are not found during catalytic pyrolysis of biomass, this implies that the conversion of anisole alone, without any other reagent, is not a sufficient model reaction to conclude on the efficiency of a catalyst for this process, but allows a better understanding of the behavior of anisole-like molecules during catalytic upgrading of pyrolysis oils.
Keywords: Anisole, Zeolites, Catalytic pyrolysis, Acid catalysis, Deactivation, Coke, Oxygenated hydrocarbons.