by Davide Lorito, Haoguang Li, Arnaud Travert, Françoise Maugé, Frédéric C. Meunier, Yves Schuurman, Claude Mirodatos
https://doi.org/10.1016/j.cattod.2017.06.041
Highlights
- Combined DRIFTS and SSITKA analyses boost methanation mechanism understanding.
- Original methanation kinetic modeling is based on physically meaningful parameters.
- Coke deposition, Ni sintering and bio-syngas poisons combine as deactivating agents.
- Kinetic modeling as a guideline for further development of bio-syngas methanation.
Abstract
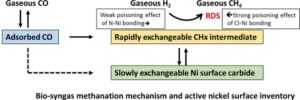
A combined operando DRIFT and SSITKA study of the methanation reaction over Ni/alumina catalysts is proposed in the absence and in the presence of various poisons representative of bio-mass gasification into syngas. In a detailed kinetic model derived from the direct inventory of the reacting surface, the rate of hydrogenation is shown to be controlled by the probability for a dihydrogen molecule to collide with an active site constituting two adjacent Ni atoms free from adsorbed CO, and free from adsorbed poisons. From this analysis, N-containing poisons (ammonia, acetonitrile) exhibit a rather mild poisoning effect, while Cl-containing molecules such as trichloroethylene are strongly but reversibly decreasing the methanation activity. No specific effects were observed for tar-like representatives such as toluene or other aromatic molecules.
Keywords
DRIFTS, SSITKA, Methanation, Bio-syngas kinetic modeling, Acetonitrile, Trichloroethylene, Catalyst deactivation